Exploiting
ionic changes in the epileptic brain to increase seizure threshold with oral supplementation
of magnesium chloride
Epilepsy is a
serious neurological disorder marked by sudden recurrent episodes of sensory
disturbance, loss of consciousness, or convulsions, associated with abnormal
electrical activity in the brain. Seizures are associated with neuronal ionic changes
(Schwartzkroin, 1998) that can be managed with increased efficacy through
magnesium chloride supplementation. Magnesium
supplementation should be part of an essential component of a management
strategy for any type of epilepsy to raise seizure threshold and maintain trace
element homeostasis.
The form of
magnesium that has been used most clinically and has the longest therapeutic
use to treat epilepsy in the literature is magnesium sulphate, however this is
not practical or convenient for patients with epilepsy. While there needs to be
more research into other forms of magnesium to manage epilepsy, there remains adequate
evidence to suggest that oral magnesium, particularly magnesium chloride, has
numerous benefits to increase seizure threshold by modulating neuronal
activity. Magnesium chloride is easy to ingest, convenient to apply, acts
effectively as a central nervous system depressant, improves insulin sensitivity,
and lowers plasma blood glucose. This essay will explain how these mechanisms
can effectively contribute towards raising seizure threshold for patients with all
types of epilepsy.
‘Non-synaptic mechanisms’ are an important
consideration in an attempt to manage neuronal activity in epilepsy. This
includes, but is not exclusive to ionic interactions that involve magnesium,
potassium, sodium and calcium. Increases in the extracellular concentration of
potassium in particular is of vital importance in order to consistently manage
epileptic seizures however this is dependent on many cofactors.
Magnesium is involved with over 300 enzymatic
reactions and has numerous mechanisms of action that make it an effective
standalone treatment to modulate neuronal excitability with great efficacy and
safety. ‘Regulation of ionic balance is a critical process that involves a
complex array of molecules for moving ions into and out of brain cells-both
neurons and glia’ (Schwartzkroin, 1998). Studies show that concentrations of
copper, magnesium, and zinc are typically altered in the epileptic brain,
particularly in those who receive anti-epileptic drugs (Saghazadeh et al., 2015) and
this decreases seizure threshold. Anti-epileptic drugs will also contribute to
a number of other imbalances such as vitamin D deficiency (Ali et al., 2004) that
can trigger breakthrough seizures through disruption of trace element
homeostasis which would require patients to increase medication as a result.
Vitamin D deficiency in itself disrupts natural homeostatic mechanisms of
calcium which can be managed not only through vitamin D supplementation (Teargarden, Meador and Loring, 2014) but also
magnesium supplementation (Levine and Coburn, 1984).
The imbalance caused by these ionic changes in the
epileptic brain, and influenced even more with anti epileptic drugs, can result
in breakthrough seizures and a subsequent increase in medication as a
questionable attempt to resolve the issue. Doctors have been accused of being
unaware of these associative deficiencies, with less than 10% of neurologists
recommending patients to take calcium and vitamin D to protect against the side
effects of these drugs (Pack, 2008). The result would be an increase of
their typical undesirable sedative side effects, bone mineral loss and possible
further deficiencies. Certain drugs, such as Lamotrogine, do not possess
sedating effects, but can disrupt circadian rhythm regulation mechanisms
causing possible breakthrough seizures by inadequate functioning of melatonin
from the pineal gland (Nzwalo et al., 2016, Quigg et al., 2016). The
rhythmicity of these seizures are often associated with bimodal peaks of occurrence,
particularly attributed to focal epilepsies, and specifically observed in
temporal lobe epilepsy (Nzwalo et al., 2016), can potentially be mitigated via
targeted therapeutic dose of oral magnesium chloride before seizure activity
occurs.
Potassium, sodium, and calcium ratios are also
important considerations as has been established in the literature over many
years (Heinemann et al, 1986), however achieving adequate magnesium status is
likely a greater challenge as our diets are likely deficient and there are
numerous mechanisms of actions that will interact beneficially with these other
elements that can be more easily attained through diet.
 |
| 'A slow potassium current (IsAHP) activated by the influx of calcium into the pyramidal neurons, plays a key role in controlling the repetitive firing of neurons throughout the brain.' http://pnbfiles.uconn.edu/PNB_Base/about/staff/facultysites/tasso/research.html |
Magnesium is an abundant mineral in the body
serving many biochemical functions and it acts as an essential electrolyte for
all living organisms. Magnesium deficiency decreases seizure threshold in human
and animal models (Osborn et al, 2015) and studies have repeatedly shown that
people with epilepsy have lower magnesium than people without epilepsy. Normal
dietary magnesium intake is estimated to be 300–350 mg per day for adults but intake
is still too low even amongst the general ‘healthy’ population. To make matters
worse, hypomagnesia is associated with epilepsy (Capellarri, 2016, de Baaij,
2015) and a wide range of medical conditions. As a result, it is not a surprise
that magnesium supplementation has been shown to be beneficial in the treatment
of many neurological conditions in particular, including preeclampsia,
migraine, and depression (Jeroen et al, 2015)
It is clear that magnesium supplementation is
essential for most people at present and moreso for those with any type of
epilepsy. As with most neurological conditions, a compromised blood brain
barrier will increase the need for magnesium. It has also been shown in a number of cases to be
more effective than medication alone and it may be possible to replace standard
medication completely if trace element homeostasis is achieved for drug
resistant epilepsy (Yuen and Sander, 2012, Abdelmalik, Politzer and Carlen, 2012, Mason
et al, 1994).
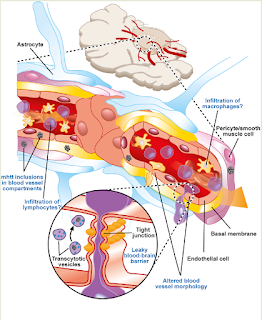 |
| A compromised blood brain barrier, associated with many conditions of the brain, results in a greaterdemand for magnesium to maintain trace element homeostasis http://journal.frontiersin.org/article/10.3389/fncel.2014.00232/full |
Magnesium comes in many forms as it needs
to be bound to certain molecules for optimal ingestion so that it can get to
the brain and central nervous system. Effectiveness for the therapeutic use
required depends on bioavailability and desired response. Magnesium citrate,
for example, has proven to be a very effective laxative, but may not be the
most efficacious from for seizure management due to poor bioavailability and
less taken up by the CNS.
It is
interesting how magnesium citrate may not help manage seizure activity
effectively (and could even trigger seizures-possibly due to poor absorption). Perhaps
surprisingly, magnesium citrate should probably be avoided for most as it
interferes with ceruloplasmin and can cause iron dysregulation as well as other
health issues. The reason this form is worth mentioning as it is commonly used
for children on strict ketogenic diets to manage constipation as magnesium
citrate has a laxative effect. Magnesium chloride can cause patients to retain
water, however potassium citrate, which is often used by patients on ketogenic
diets to alkalise the urine for kidney health, can help to release these fluids
and relieve this discomfort.
It is important to consider the mechanisms of
action for magnesium. Seizures occur when the central nervous system becomes
overstimulated. Magnesium can act as an effective CNS depressant, with
numerous beneficial functions intracellularly and extracellularly to supress
seizure activity to modulate neuronal excitability. The primary mechanism is
most likely its ability to antagonize excitation through the
N-methyl-d-aspartate receptor (Yuen and Sander, 2012).
 |
| 'The primary mechanism is most likely its ability to antagonize excitation through the N-methyl-d-aspartate receptor' IMG- http://webvision.med.utah.edu/book/part-v-phototransduction-in-rods-and-cones/glutamate-and-glutamate-receptors-in-the-vertebrate-retina/ |
Most of the research regarding epilepsy comes from numerous
studies on eclampsia (Berhan and Berhan, 2015) with some detailing treatment
for status epilepticus (Tan et al, 2015). ‘Eclampsia is defined as de novo
seizure in a woman with the hypertensive complication of pregnancy known as
preeclampsia (PE), and is a leading cause of maternal and fetal morbidity and
mortality worldwide.’ (Johnson, 2015)
 |
| Magnesium Sulphate has been in clinical use for almost a centrury to treat epileptic seizures in women with pre-eclampsia. |
Any kind of epilepsy results in a compromised blood
brain barrier (Oby and Janigro, 2006). Like preeclampsia, neuroinflammation and
a compromised blood brain barrier permeability (Johnson, 2015) increase the
need for magnesium (‘leaky brain’) so a favourable response is gained from
supplementation, reversing seizure activity. We know for example, that
inflammatory molecules contribute to neuronal hyperexcitability (Lori, Frigerio
and Vezzani,
2016) which
would naturally decrease seizure threshold dramatically. Keeping blood glucose
low is neuroprotective and anti-inflammatory, magnesium can do this
effectively.
Similar to the ketogenic diet, magnesium has had nearly a
century of clinical use to treat epilepsy. It been used as prophylaxis and
treatment of seizures associated with eclampsia however because of the
availability of well studied anticonvulsant drugs it has not been
tested widely in the treatment of epileptic seizures (Abdelmalik, Politzer and
Carlen, 2012, Mason et al, 1994). As
with the ketogenic diet, oral magnesium acts as a natural statin to reduce
seizure threshold and keeps blood glucose low. (Guerrero-Romero et al, 2015) It
is absolutely crucial to keep blood glucose consistent as hyperglycaemia has
been shown to lower seizure threshold (Stafstrom, 2008).
In conclusion, restoration of specific ionic
changes in the epileptic brain with supplementary therapeutic doses of
magnesium chloride can be an effective strategy to increase seizure threshold.
As a stand-alone therapy, or in combination with manipulation of trace elements
and electrolytes and/or anti epileptic medications patients can attain improved
seizure control through trace element homeostasis. It remains unclear whether
these ions are causal to, or a cofactor in the development of epilepsy, however
it has been demonstrated that seizure control can be maintained through ‘non
synaptic mechanisms’, with magnesium being a key element with numerous
mechanisms of action.
Studies on magnesium chloride have demonstrated
that it can be a convenient, efficacious supplemental therapeutic aid to
increase seizure threshold. Magnesium chloride has shown to ingest efficiently
due to its composition and its bioavailability is favourable. Taken orally it
can assist in maintaining trace element homeostasis to either complement or replace
drugs which can cause numerous trace element deficiencies. It is convenient and
possibly the most efficient form of magnesium for ingestion as minerals need to
be dissolved in gastric acid to go into the solution. Magnesium chloride has
extra chloride molecules to produce hydrochloric acid in the stomach which
enhances its absorption. It is pertinent to note that interaction with other
trace elements needs to be taken into account however supplementation with
magnesium chloride could provide patients with the most effective method on its
own to better prevent neuronal hyperexcitability through its many promising
mechanisms of action.
References
Abdelmalik, P. A., Politzer, N. and Carlen, P.L. (2012). Magnesium
as an effective adjunct therapy for drug resistant seizures. The Canadian Journal of Neurological
Sciences. 39(3):323-7. Available from
http://www.ncbi.nlm.nih.gov/pubmed/22547512 [Accessed 02 February 2016]
Ali, F.
E. et al. (2004). Loss of Seizure Control Due to Anticonvulsant-Induced
Hypocalcemia. The Annals of Pharmacotherapy, 38(6):1002-5.
Available from http://www.ncbi.nlm.nih.gov/pubmed/15084684 [Accessed 08
February 2016]
Berhan, Y. and Berhan, A. (2015). Should magnesium sulfate
be administered to women with mild pre-eclampsia? A systematic review of
published reports on eclampsia. The Journal of Obstetrics and Gynaecology
Research, 41(6):831-42. Available from
http://onlinelibrary.wiley.com/doi/10.1111/jog.12697/full [Accessed 04 February
2016]
Cappellari, A. M. (2016). Neonatal focal seizures
and hypomagnesemia: A case report. European Journal of Paediatric
Neurology, 20(1):176-178. Available from
http://www.ejpn-journal.com/article/S1090-3798(15)00179-8/abstract [Accessed 05
February 2016]
de
Baaij, J. H. (2015). The art of magnesium transport. Magnesium Research,
28(3):85-91. Available from http://www.ncbi.nlm.nih.gov/pubmed/26446763
[Accessed 07 February 2016]
Guerrero-Romero, F. et al. (2015).
Oral magnesium supplementation improves glycaemic status in subjects with
prediabetes and hypomagnesaemia: A double-blind placebo-controlled randomized
trial. Diabetes and Metabolism. 41(3):202-7.
Available from https://www.researchgate.net/publication/275589606_Oral_magnesium_supplementation_improves_glycaemic_status_in_subjects_with_prediabetes_and_hypomagnesaemia_A_double-blind_placebo-controlled_randomized_trial
[Accessed 04 February 2016]
Heinemann,
U. et al. (1986). Extracellular calcium and potassium concentration changes in
chronic epileptic brain tissue. Advances in Neurology, 44:641-61. Available from http://www.ncbi.nlm.nih.gov/pubmed/3518350
[Accessed 04 February 2016]
Levine, B. S. and Coburn J. W.
(1984). Magnesium, the Mimic/Antagonist of Calcium. The New England
Journal of Medicine, 310:1253-1255.
Available from http://www.nejm.org/doi/full/10.1056/NEJM198405103101910
[Accessed 08 February 2016]
Lori, V., Frigerio, F. and
Vezzani, A. (2016). Modulation of neuronal excitability by immune mediators in
epilepsy. Current Opinion in Pharmacology, 26:118-23.
Available from http://www.ncbi.nlm.nih.gov/pubmed/26629681 [Accessed 06
February 2016]
Saghazadeh, A. et al. (2015) Possible role of trace elements in epilepsy and febrile
seizures: a meta-analysis. Nutrition Reviews, 73(11):760-779. Available from http://nutritionreviews.oxfordjournals.org/content/73/11/760.abstract
[Accessed 07 February 2016]
Jeroen H. F. et al. (2015). Magnesium in Man: Implications
for Health and Disease. 95(1):1-46. Available from
http://physrev.physiology.org/content/95/1/1 [Accessed 02 February 2016]
Johnson, A. C. (2015) Mechanisms
of Seizure during Pregnancy and Preeclampsia.
Graduate
College Dissertations and Theses. Paper 336. Available from
http://scholarworks.uvm.edu/cgi/viewcontent.cgi?article=1335&context=graddis
[Accessed 04 February 2016]
Mason, B.
A. et al. (1994). Magnesium is more efficacious than phenytoin in
reducing N-methyl-D-aspartate seizures in
rats. American Journal of Obstetrics and Gynaecology Research, 171(4):999-1002. Available
from
https://www.researchgate.net/publication/15256355_Magnesium_is_more_efficacious_than_phenytoin_in_reducing_N-methyl-D-aspartate_seizures_in_rats_Am_J_Obstet_Gynecol
[Accessed 04 February 2016]
Nzwalo, H. et al. (2016). 24- hour rhythmicity of seizures
in refractory focal epilepsy. Epilepsy and Behaviour, 55: 75-78. Available from
http://www.sciencedirect.com/science/article/pii/S1525505015006605 [Accessed 20
March 2016]
Oby, E. and Janigro, D. (2006). The blood-brain
barrier and epilepsy. Epilepsia, 47(11):1761-74.
Available from http://www.ncbi.nlm.nih.gov/pubmed/17116015 [Accessed 04
February 2016]
Osborn, K. E. et al.
(2015). Addressing potential role of magnesium dyshomeostasis to improve
treatment efficacy for epilepsy: a reexamination of the literature. Journal of Clinical
Pharmacology. 56(3):260-5. Available from
http://onlinelibrary.wiley.com/doi/10.1002/jcph.626/full [Accessed 07 February
2016]
Pack, A. (2008). Bone health in people with
epilepsy: Is it impaired and what are the risk factors?. Seizure, 17(2):181-6. Available from
http://www.ncbi.nlm.nih.gov/pubmed/18187347 [Accessed 04 February 2016]
Quigg, M. et al. (2016). Insomnia in epilepsy is associated
with continuing seizures and worse quality of life. Epilepsy Research,
Available from
http://www.sciencedirect.com/science/article/pii/S0920121116300328 [Accessed 20
March 2016]
Rodríguez-Morán, M. and Guerrero-Romero, F. (2003). Oral
magnesium supplementation improves insulin sensitivity and metabolic control in
type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes
Care, 26(4),1147-52. Available from http://care.diabetesjournals.org/content/26/4/1147.full
[Accessed 04 February 2016]
Saghazadeh et al. (2015). Possible
role of trace elements in epilepsy and febrile seizures: a meta-analysis.
Nutrition Reviews, 73(11):760-79. Available from
http://nutritionreviews.oxfordjournals.org/content/early/2015/10/02/nutrit.nuv026
[Accessed 04 February 2016]
Schwartzkroin, P. A., Baraban, S. C. and Hochman, D. W. (1998). Osmolarity, ionic flux, and changes in brain
excitability. Epilepsy Research, 32(1-2):275-285. Available from
http://www.sciencedirect.com/science/article/pii/S0920121198000588 [Accessed 09
February 2016]
Stafstrom,
C. E. (2008). Hyperglycemia Lowers Seizure Threshold. Seizure, 17(2):181-6. Available from
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC387262/ [Accessed 07 February 2016]
Tan, W.
W. et al. (2015). Use of Magnesium Sulfate Infusion for the Management of
Febrile Illness-Related Epilepsy Syndrome A Case Series. Child Neurology Open,
January - March 2015: 1-5. Available from
http://cno.sagepub.com/content/2/1/2329048X14550067.full.pdf [Accessed 04
February 2016]
Teargarden, D. L., Meador, K. J. and Loring, D. W.
(2014). Low vitamin D levels are common in patients with epilepsy. Epilepsy
Research, 108(8):1352-6. Available from
http://www.ncbi.nlm.nih.gov/pubmed/25060996 [Accessed 04 February 2016]
Yuen, A. W. and Sander, J. W.
(2012). Can magnesium supplementation reduce seizures in people with epilepsy?
A hypothesis. Epilepsy Research. 100(1-2):152-6.
Available from http://www.ncbi.nlm.nih.gov/pubmed/22406257 [Accessed 02
February 2016]





